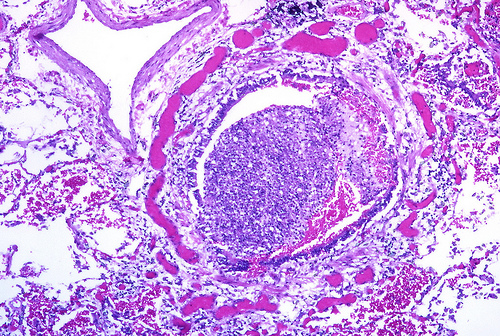
Pandemic H1N1 (pH1N1), as has been widely documented, has had a greater burden on younger populations. In a normal flu season, 90% of deaths are in persons over age 65. With pH1N1 the percentages have been nearly reversed with 88% of deaths occuring in persons under 65.
Overall pH1N1 has exibited low pathogencity, resulting in approximately 12,000 (estimated) deaths in the US. This is well below the widely quoted figure of 36,000 annual deaths from seasonal flu.
The World Health Organization (WHO) has described pH1N1 as a moderate” pandemic. Many flu experts have never been comfortable with qualitative terms such as “mild” or “moderate”, perhaps in part because they also minimize the import of the disease, and can dissuade publics from taking protective action.
Researchers from the National Institites of Health (NIH) have devised what they term is an “apples-to-apples” methodology to estimate the burden of disease on a population. The metric, termed “Years of Life Lost” (YLL), takes into account the age-distribution of disease-related deaths and extrapolates potential years of life lost across age groups. Expressed as a function, the higher the numbers of deaths, and the lower the mean-age of deaths, the higher is YLL.
NIH researchers describe the impact of pH1N1 as “significant.” By their YLL metric, the burden of p1N1 exceeds that of typical seasonal flu dominated by Influenza A H3N2 virus. NIH research suggests pH1N1 may have had a comparable burden to the (Hong Kong) flu of 1968 (also described as a relatively mild pandemic).
There perhaps is no perfect way to measure the scope, intensity, and duration of a disease outbreak. Numbers of deaths, for obvious reasons, is a metric that is intuitively understood, and of course makes for headlines and optics. While YLL provides a relatively simple metric to compare impacts across pandemics, the measure is still fundametally sensitive to numbers of deaths. Overall, YLL estimates still suggest a moderate burden of disease for pH1N1.
CIDRAP: Study: In life-years lost, H1N1 pandemic had sizable impact
PLoS Currents: Preliminary Estimates of Mortality and Years of Life Lost Associated with the 2009 A/H1N1 Pandemic in the US and Comparison with Past Influenza Seasons
Tagged: flu, h1n1, influenza, International News, pandemic flu, swine flu, US News