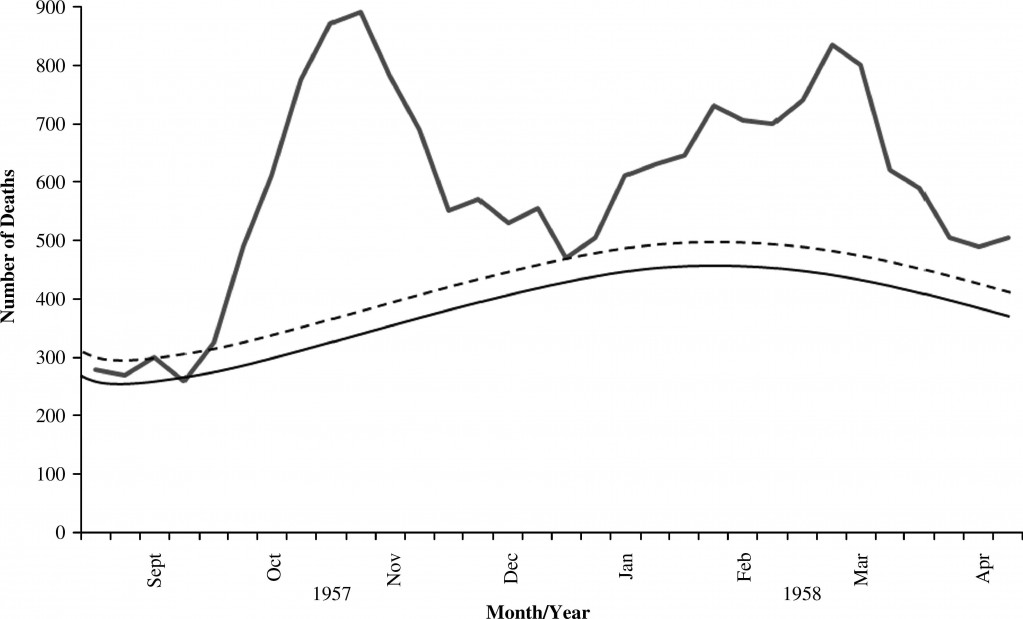
CIDRAP reports that British researchers published one of the first large serologic studies of pandemic H1N1 infection. Serology typically involves a blood test to detect the presence of antibodies against an infecting microorganism.
According to experts, serologic studies provides a sharper picture of the burden of disease than is available from flu-surveillance, and this helps public health planners better assess vaccination efforts.
KEY research findings were:
In regions with high H1N1 incidence, as many as one in three children under 15 years of age were infected with H1N1 in the first wave of infections.
- This was ten times higher than official estimates derived from flu surveillance.
A substantial proportion of older adults had preexisting immunity to H1N1, which may have resulted from prior exposure to “antigenically similar” flu viruses.
- Depending upon the techniques employed, around one in four adults aged 65 or older (or as many as two in three) should be protected from H1N1.
Editor’s Comment: These findings strongly underscore the risks of H1N1 to young populations; they should remain a priority target group for vaccination, The findings suggest some upper and lower bounds on pre-existing immunity to H1N1 for seniors. However, it is fair to say a significant proportion of seniors are at risk.
Tagged: flu, h1n1, influenza, swine flu, Vaccine Updates